PU Guidelines
The most recent guideline was released: 15 November 2019
Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline Prevention Chapters are available at
www.internationalguideline.com.
Introduction
The first edition of the guideline was developed as a two-year collaboration between the National Pressure Injury Advisory Panel (NPIAP) and the European Pressure Ulcer Advisory Panel (EPUAP). In the second edition of the guideline, the Pan Pacific Pressure Injury Alliance (PPPIA) joined the NPIAP and EPUAP.
For the 2019 edition, the EPUAP, NPIAP and PPPIA were joined by 14 international Associate Organizations, 168 international pressure injury experts working in small working groups, 699 stakeholders and over 1200 patient consumers and their caregivers.
The third edition of the guideline was released in November 2019. The goal of this international collaboration was to develop evidence-based recommendations for the prevention and treatment of pressure injuries that could be used by health professionals throughout the world. An explicit scientific methodology has been used to identify and critically appraise all available research.
The 2019 version contains an updated review of the research literature and recommendations that reflect the most recent evidence. It provides a detailed analysis and discussion of available research, critical evaluation of the assumptions and knowledge in the field, recommendations for clinical practice, good practice statements, implementation considerations, a description of the methodology used to develop the guideline and acknowledgements of the many experts formally involved in the development process.
Download the Full 2019 Guideline Translations
Download the Czech Translation of the Guideline
FREE DOWNLOADDownload the Arabic Translation of The Guideline
FREE DOWNLOADDownload the 2019 Quick Reference Guide Spanish Translation version.
Download the 2019 Quick Reference Guide German Translation version.
Download the 2019 Quick Reference Guide Slovak Translation version.
Download the 2019 Quick Reference Guide Malaysian Translation version.
Download the 2019 Quick Reference Guide Danish Translation version.
Download the 2019 Quick Reference Guide French Translation version.
Download the 2019 Quick Reference Guide Italian Translation version.
Download the 2019 Quick Reference Guide Portuguese Translation version.
Download the 2019 Quick Reference Guide Brazilian Translation version.
Download the 2019 Quick Reference Guide Turkish Translation version.
Download the 2019 Quick Reference Guide Swedish Translation version.
Download the 2019 Quick Reference Guide Finnish Translation version.
Download the 2019 Quick Reference Guide Dutch/Belgium version.
Download the 2019 Quick Reference Guide Greek version.
Download the 2019 Quick Reference Guide Simplified Chinese version.
Download the 2019 Quick Reference Guide Croatian version
Download the 2019 Quick Reference Guide Thai Translation version.
Download the 2019 Quick Reference Guide Czech Translation version.
Download the 2019 Quick Reference Guide Arabic Translation version.
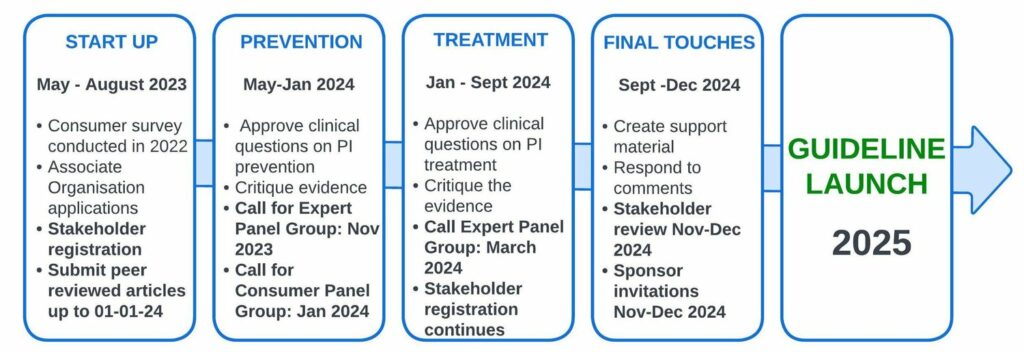
2025 Guideline Development
The Fourth Edition of the International Pressure Ulcer/Injury Guideline is currently in progress, and it is expected to be published on STOP PU Day 2025. Stay informed about our updates and advancements by becoming a guideline stakeholder through registration, attending our presentations at pressure ulcer/injury conferences from 2023 to 2025, and keeping up with the Member Organizations on social media.
Get Involved
Associate Organizations
The collaboration with Associate Organizations is greatly appreciated by NPIAP/EPUAP/PPPIA in the creation of past Guideline editions. We will soon disclose the wound organizations involved as Associate Organizations in the development of the 2025 edition of the Guideline.
Panel Group Members
The NPIAP/EPUAP/PPPIA greatly appreciate wound experts’ contributions to past guideline editions through Small Working Groups. The Guideline Governance Group will soon invite wound experts and patient/informal caregivers to express interest in joining topic-focused Panel Groups for the 2025 guideline development. Nominations are expected to open in late 2023.
Stakeholders
The NPIAP/EPUAP/PPPIA greatly appreciates the input from individuals who reviewed past Guideline editions. The Guideline Governance Group uses a stakeholder mailing list to share updates on ongoing Guideline development.
Researchers
A comprehensive database search is conducted to find peer-reviewed evidence, which is then assessed against inclusion criteria for relevance to PICOT clinical questions. Appropriate evidence meeting the criteria is evaluated, and data is extracted for synthesis in the Guideline. Accepted research published in peer-reviewed journals between September 2018 and January 2024 can be submitted.
More news related to the 2025 edition can be found HERE.